A PATIENT WITH VENTRICULAR TACHYCARDIA IS WEARING A VEST OF ELECTRODES DURING A NONINVASIVE HEART-MAPPING PROCEDURE CALLED ELECTROCARDIOGRAPHIC IMAGING.
BY DARCY LEWIS
Every day, nearly 1,000 Americans die from sudden cardiac arrest, a catastrophic event in which the heart suddenly stops functioning. The most common cause of sudden cardiac arrest is a sustained, super-fast heart rhythm called ventricular tachycardia, commonly called V-tach. Among heart specialists, this condition is known as VT.
WITHIN A SINGLE BEAT, WE CAN VISUALIZE IN DETAIL THE PATH THE ELECTRICITY TAKES AND THE SHAPE AND SIZE OF THAT PATIENT’S HEART.
During VT, the heart produces irregular electrical signals that cause its ventricles, or lower chambers, to beat so rapidly that they can’t pump enough blood to the body. If ventricular tachycardia lasts for only a few seconds, the result may be a feeling of lightheadedness or breathlessness. Some people lose consciousness.
But if ventricular tachycardia lasts longer than a few seconds, the heart may stop.
“It’s important to emphasize that when most people say they feel their heart race or skip a beat, it’s very unlikely to be ventricular tachycardia,” says Phillip Cuculich, MD, Washington University cardiologist at Barnes-Jewish Hospital. “We don’t want to scare people. But what’s so difficult about this most serious of arrhythmias is that it affects both young and old, typically without warning.”
What comes next for VT survivors?
Only 10% of people who experience a sudden cardiac arrest or a prolonged episode of ventricular tachycardia survive. But survivors also face new worries: What went wrong with their heart and, more importantly, can doctors prevent it from happening again?
VT typically is caused by scar tissue in the heart, Cuculich says. “For most people in the United States who develop VT, a prior heart attack has left some scarring,” he says. “Eventually, the stiff, thickened scar disrupts the heart’s normal electrical patterns and VT occurs.” Cardiac scarring can also develop in people who have had heart failure; their risk of developing VT is much higher than in the general population.
As for preventing a future cardiac arrest, that’s where an implantable cardiac defibrillator, or ICD, comes in. “An ICD is smaller than a deck of cards and is implanted underneath the skin, with leads attached to the heart that measure its rhythm,” says Cuculich. “If the heart ever goes into another life-threatening rhythm, the ICD will automatically deliver a life-saving jolt of electricity to restore a normal rhythm.”
Treating VT
For many people with ventricular tachycardia, treatment involves cardiac ablation, an inpatient procedure that uses carefully directed heat energy to create tiny new scars on the heart. These scars can avert erratic heart rhythms.
During cardiac ablation, which is performed using general anesthesia, a cardiologist threads one or more long, narrow tubes called catheters—often starting in the groin area—into a vein or veins, gently guiding them to the heart. Each catheter includes sensors on its tip that record the heart’s electrical patterns and can send controlled electrical impulses that are used to create the new, beneficial scars.
Once the catheters reach the heart, the cardiologist induces ventricular tachycardia, then uses the catheters to gather information about the heart’s electrical signals. “We need to understand where the circuitry is moving through the existing scar,” Cuculich says. “If we can find the circuit’s critical component, we can cauterize that exact location inside the heart and destroy that circuit.”
When cardiac ablation works, it tends to work very well, freeing patients from VT. But ablation can take up to seven hours to complete, and inpatient recovery can take several days. However, for many patients who undergo cardiac ablation, the procedure does not provide an effective solution, in good part because of the limitations of the technology.
“The heart can be up to 20 millimeters thick, but our catheters can only reach to 3.5 millimeters deep, meaning we can’t always send the ablative heat deep enough into the tissues to treat the circuit,” Cuculich says.
Room for improvement
Cuculich, who specializes in cardiac electrophysiology, has devoted his career to developing safer, more effective solutions for dangerous arrhythmias like VT. “At the Washington University and Barnes-Jewish Heart & Vascular Center, we take a patient-centered view,” he says. “How can we make this better for the people we treat?”
Cuculich singles out two innovations that are available now at Barnes-Jewish Hospital, as well as a third option that is under development.
One such innovation that Cuculich and colleagues have employed is the use of an MRI scan, which is done before the ablation and used during the procedure. This noninvasive scan uses radio waves and magnets to create detailed images of the heart—including scar tissue—in two or three dimensions. “The MRI shows us where the scar is ahead of time, so we don’t spend precious minutes during the ablation to make that discovery while the patient is in VT,” he says. “We use the information the MRI offers to perform the ablation as efficiently as possible.”
Another innovative option, called electrocardiographic imaging, also produces a noninvasive map of the heart that can help guide cardiac ablation. During this imaging procedure, the patient wears a special vest containing 252 electrodes that capture electrophysiological data from the patient’s body surface while the patient undergoes a CT scan. The data from the electrodes and the scan are combined to create 3D cardiac maps.
The maps reveal crucial information about the size and shape of the patient’s heart and how it generates electricity. “When we initiate an abnormal heart rhythm in the patient, the vest of electrodes captures an immediate, panoramic picture of the heart’s electrical signals,” Cuculich says. “Within a single beat, we can visualize in detail the path the electricity takes and the shape and size of that patient’s heart, and we can discover the exact source of the arrhythmia.”
Noninvasive cardiac radioablation
The net result of incorporating pre-ablation MRIs and noninvasive electrode mapping, says Cuculich, is that a procedure that used to take five to seven hours now takes about three. But he believes there is still room for improvement.
“We have the scar map from the MRI, and we have a map showing the path of electricity, which means we should know where the arrhythmia is coming from,” he said. “And that should mean we can noninvasively target that area of the heart with focused radiation.”
Cuculich and Clifford Robinson, MD, a Washington University radiation oncologist, recently teamed up to develop a new approach for treating ventricular tachycardia, one that cardiologists nationwide are saying is a game-changer for patients with few remaining options. Robinson typically uses radiation therapy when he treats people with cancer at Siteman Cancer Center, based at Barnes-Jewish Hospital and Washington University School of Medicine. But he and Cuculich have shown that radiation therapy—aimed directly at the heart—can successfully treat patients with VT.
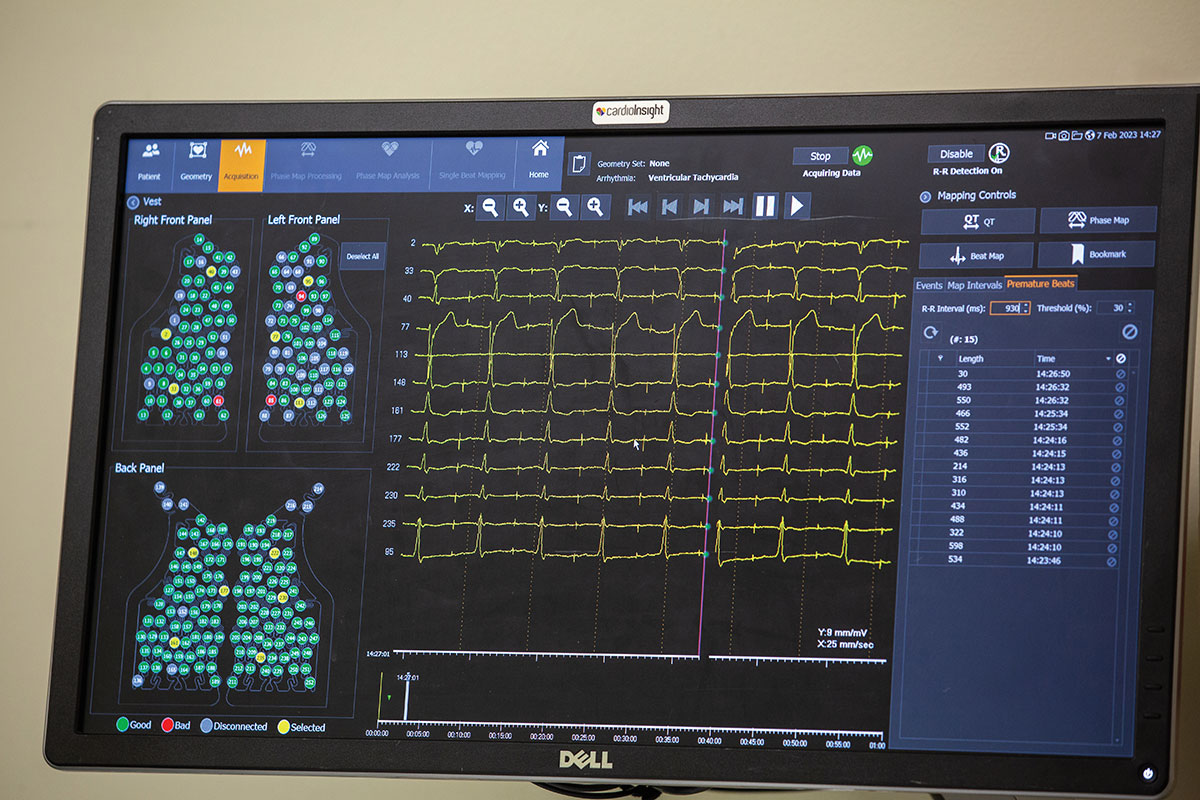
ELECTROCARDIOGRAPHIC IMAGING USES A VEST THAT CONTAINS 252 ELECTRODES, EACH OF WHICH CAPTURES DATA ABOUT THE HEART TO CAPTURE A 3D MAP.
Clifford and Robinson have published several proof-of-concept studies, including one in the New England Journal of Medicine, demonstrating the promise of this noninvasive treatment for VT. As a result, the procedure will be further evaluated in an international, randomized clinical trial. Washington University School of Medicine will participate as one of the trial sites, with cardiologist Dan Cooper, MD, as the site’s principal investigator.
The treatment uses focused radiation in much the same way cancer therapy uses it to shrink tumors without surgery or chemotherapy. Cuculich says, “We have learned how to focus radiation into the scarred parts of the heart, essentially treating that area in the same manner a cancer tumor is treated.”
In noninvasive cardiac ablation, Cuculich says, a patient “lies down on the table and listens to one or two songs through headphones while the gantry—the machine that delivers radiation—moves around them, delivering focused radiation to the part of the heart that contains the scar. The patient then stands up and leaves,” Cuculich says. “This technique is turning a seven-hour invasive procedure into a noninvasive, seven-minute procedure.”
Cardiologists also are continuing to search for new ways to improve the standard cardiac ablation procedure. One potential improvement, known as multipolar mapping, involves using up to 20 sensors on the tip of each catheter. The sensors can be arranged in different formations, with the goal of capturing data more efficiently to lessen the time each patient must spend in arrhythmia during the procedure.
Another advance in treatment involves using different types of energy to ablate the heart scars. The goal in this case is to render the scar electrically inert and unable to disrupt the heart’s rhythm, says Cuculich. Other researchers have developed a catheter whose tip contains a needle. It lets the cardiologist send energy deeper into the heart muscle than a conventional catheter does.
But all these innovations are designed to improve treatment options for cardiac ablation, a treatment that finds scarred heart tissue, then uses energy to destroy parts of it. “The downside to destroying parts of the heart is that we need the heart because it sustains life,” says Cuculich. “Nobody wants to cause additional damage to the heart, but right now we don’t have other viable treatment options. We’re working toward a noninvasive treatment that doesn’t damage the heart.”