JEFFREY CRIPPIN, MD, GASTROENTEROLOGIST AND HEPATOLOGIST, DISCUSSES TREATMENT OPTIONS WITH A PATIENT.
Photography by Gregg Goldmann
BY PAM MCGRATH
Although its importance is often unappreciated, the liver has far-reaching effects on almost every organ system in the body. And its singular ability to regenerate makes living-donor transplantation a life-altering option for those in whom this vital organ is failing.
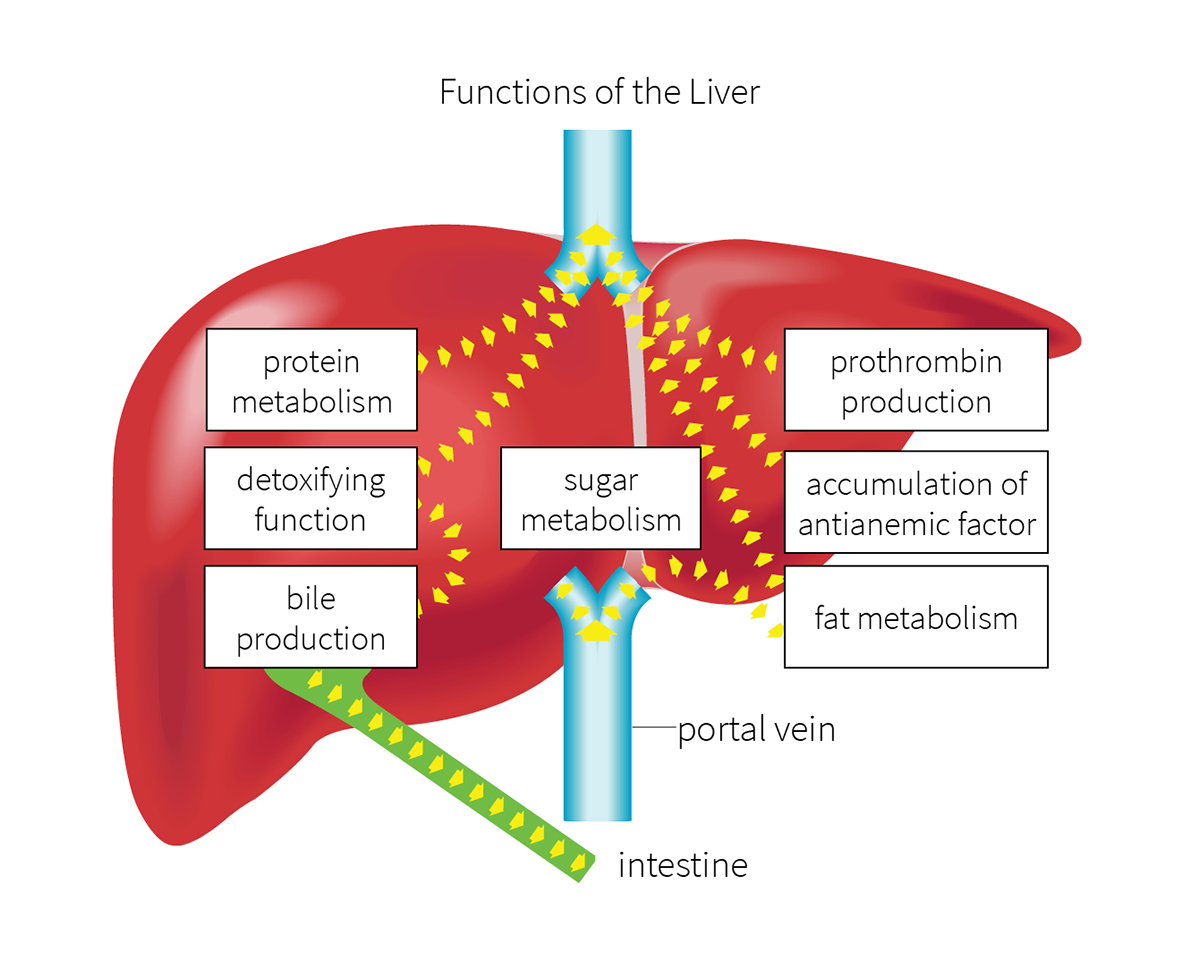
Illustration courtesy of Shutterstock
In Greek mythology, the god Zeus punished the Titan Prometheus for giving humans the gift of fire by tying him to a rock and having an eagle eat his liver. Every day, Prometheus’ liver regrew, and every day the eagle returned to feast.
Although the penalty Zeus imposed on Prometheus is gruesome, it does provoke some interesting questions: Did the Greeks know about the liver’s ability to regenerate? And is that why they regarded it as the seat of life, the center of the soul?
While the extent of the Greeks’ knowledge of the liver remains unclear, its purpose—and its ability to regenerate—have been carefully studied by physicians and scientists. For many of us, however, the liver’s function within the body is a puzzlement.
A powerhouse of metabolic activity
“Most patients I talk with don’t really know what the liver does—until it doesn’t,” says Jeffrey Crippin, MD, WashU Medicine gastroenterologist and hepatologist at Barnes-Jewish Hospital. “It performs an estimated 5,000 tasks that play a major role in all of the body’s metabolic processes.”
After the food we eat is digested by the stomach and intestines, those byproducts are absorbed into the bloodstream, which carries them to the liver. The liver filters the blood as it arrives, identifying nutrients that can be converted into materials the body can use. It stores these materials, releasing them into the circulatory system when they are needed by cells. The liver also identifies toxins in the blood and filters them out through urine and stool.
“The liver stores energy in the form of sugars,” Crippin says. “Because we don’t eat constantly to replenish our energy, we need to store energy until it’s needed. Otherwise, we wouldn’t be able to do even simple things like getting out of bed in the morning,” says Crippin. “The liver serves as the body’s fuel tank.” And it disperses fuel in a way that keeps the level of sugar in the bloodstream constant.
The liver also produces bile, which breaks down and absorbs the fats that we eat. This process enables the liver to convert and store carbohydrates and protein for future use, and to synthesize other types of fat.
“The liver is a workhorse. It manufactures cholesterol, triglycerides and proteins, some of which are important in controlling how well the blood clots,” says Crippin. “Liver problems arise when the organ can’t complete its job of storing energy, filtering nutrients and toxins, and manufacturing other elements needed by the body.”
When the liver malfunctions
In the past several years, Crippin notes, fatty liver disease has become the most prevalent cause of liver dysfunction. Although this condition can be caused by overconsumption of alcohol, the more common causes are related to obesity, diabetes, high cholesterol and high blood pressure. “Fatty liver disease is usually associated with a metabolic disorder, in which fatty tissue accumulates in the liver, causing its filtering system to become overloaded,” he says.
Fatty liver disease can be controlled—and sometimes reversed—through weight loss, lowering cholesterol and triglycerides, avoiding alcohol and controlling diabetes. Hepatitis C, caused by a virus, can also damage the liver, as can rare metabolic disorders, including hemochromatosis, an iron overload; Wilson’s disease, a copper overload; and a number of autoimmune disorders that involve the liver.

WILLIAM CHAPMAN, MD TRANSPLANT SURGEON PERFORMS LIFE-CHANGING LIVER TRANSPLANT SURGERY AT BARNES-JEWISH HOSPITAL.
When the liver isn’t working effectively, early diagnosis can make a difference in outcomes. A liver specialist, called a hepatologist, can diagnose liver disease and determine the best treatment options. “Unfortunately,” Crippin notes, “in the early stages of liver disease, people often don’t experience symptoms or, if they do, the symptoms aren’t associated with liver disease.” For example, damaged bile ducts in the liver can cause itching, which may be attributed to some cause other than liver disease.
When left untreated, liver disease can cause scar tissue that replaces healthy liver cells—a condition called cirrhosis that can lead to significant health consequences. “In the case of advanced liver disease,” Crippin says, “transplantation may be the best treatment option.”
Liver transplantation
The first successful liver transplant in Missouri was performed by WashU Medicine transplant surgeons at Barnes-Jewish Hospital in 1985. According to WashU Medicine transplant surgeon William Chapman, MD, FACS, approximately 9,000 liver transplants are performed in the U.S. annually. “But at any point during a given year, there likely will be at least 10,000 people waiting on the transplant list, a number far greater than the number of donor livers available,” he says.
For some people, a living-donor liver transplant may be a viable option. Because the liver can regenerate, a living donor can donate a portion of their liver—up to 65%--to a person with liver failure. Over time, the remaining portion of the donor’s liver will regenerate to its original size, while the transplanted portion will grow to the size needed by the recipient.
In January 2024, a transplant team from the Washington University and Barnes-Jewish Transplant Center performed its first living-donor liver transplant in 10 years at St. Louis Children’s Hospital. The recipient, a 6-month-old infant with biliary atresia, received a portion of liver donated to her by a family member who is not a blood relative.
Noting the 10-year time lapse between living-donor liver transplants at the Transplant Center, Chapman says: “We didn’t stop performing liver transplants during that time; in fact, our program grew. On average, our liver transplant team performs 150 to 175 transplants a year, 10 to 20 of them for pediatric patients at St. Louis Children’s Hospital and the remainder for adults at Barnes-Jewish Hospital.”

IN JANUARY 2024, WASHU MEDICINE TRANSPLANT SURGEONS REMOVED A SECTION OF LIVER FROM NADIA, A LIVER DONOR, AND TRANSPLANTED IT INTO HER SIX-MONTH-OLD FAMILY MEMBER, EDEN.
Chapman also notes that during that time, the Transplant Center was fortunate in that it didn’t need to use organs from living donors. “We screened many potential donors but ultimately were able to identify suitable livers from deceased donors.” He adds: “Our view is that if we can get similar results from a deceased-donor organ and avoid subjecting someone to a surgery that is not going to benefit them medically, that’s our preferred choice.”
However, securing deceased-donor organs has become more challenging because of revisions made to the scoring system that determines a person’s placement on the liver-transplant waitlist. These revisions were designed to more accurately prioritize people on the list based on medical urgency, with the goal of minimizing waitlist deaths by moving the sickest patients to the head of the line.
“In particular, these changes impact people whose livers are not failing, although they are not in the best of health. They may have poor quality of life, but their MELD scores are low,” says Chapman. A patient’s MELD score (model for end-stage liver disease) estimates the short-term risk of death in people with chronic liver disease.
A person with liver cancer, for example, may have a low MELD score and be placed low on the wait list because the liver is functioning well, despite the presence of cancer. However, Chapman notes, “the cancer will advance and result in death unless a transplant is performed.” Living-donor liver transplantation can be a viable option for a person whose position on the waitlist has been affected by the new MELD scoring system.
Potential donors, generally age 50 and younger, undergo a careful medical screening to determine their suitability for liver donation. In addition to medical screening, the Transplant Center assesses psychological stability and the level of motivation needed to recover from donation surgery. “Every potential donor works with a living-donor advocate,” Chapman says. “That specialist helps them through each step of the process.”
Because living organ donation is a significant decision, the assessment team also works to ensure that all potential donors understand they are not obligated to proceed. If approved, a donor will have the opportunity to discuss all aspects of the process, including recovery expectations and risks involved.
Improving availability of deceased-donor organs
While living-donor liver transplant is an option for some, the need for organs from deceased donors is pressing. One way to increase the number of organs available is to ensure that as many as possible are deemed viable for transplantation.
Traditionally, a liver from a deceased donor is preserved prior to transplantation using a method called static cold storage. In this process, the organ is procured and prepared, and then its temperature lowered using a cold preservation solution. Despite these efforts, some degradation of the organ occurs. Guidelines recommend that a deceased-donor liver is viable for transplantation for up to six hours, which limits the amount of time an organ can travel from the recovery site to the transplantation site.
That time limitation can also affect the scheduling of transplantation surgeries. As a result, some deceased-donor organs become unavailable to those most in need.
A new and FDA-approved development in managing deceased-donor organs, called ex-vivo normothermic machine perfusion (NMP), may help make more deceased-donor livers available. In this process, the donated liver is procured, kept at its normal body temperature and perfused with a blood-based solution that maintains the liver’s oxygen levels and ensures it remains metabolically functional.
“NMP offers a significant benefit in that it gives us up to 24 hours to perform the transplant,” says Chapman. Another advantage of NMP is that it enables transplant surgeons to test the organ’s function before it is transplanted. Such testing can reveal how well it clears toxins and utilizes glucose, and whether it produces bile, among other results.
“Because NMP allows for testing, we have the potential to identify viable organs that we might have deemed unusable in the past because we couldn’t verify their function,” Chapman notes.
Another method of preserving organs, called normothermic regional perfusion (NRP), is currently being studied at WashU Medicine. This method of organ preservation uses ECMO, or extracorporeal membrane oxygenation, to keep a deceased donor’s organs perfused with blood and oxygen while they remain with the body.
Chapman notes: “Advances such as these new perfusion techniques help make liver transplantation possible for more of the people in need.”